哈尔滨工程大学
第一章单元测试
- If a nucleus has a spin quantum number of 3, the minimum value of its spin magnetic quantum number is _______. ( )。
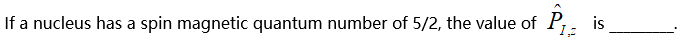 ( )。
( )。- The total angular momentum quantum number of the electrons in an atom is j. It is known that the value of j is larger than that of the spin quantum number I. When the nucleus is placed in a magnetic field, the original energy level is split into 8 sub ones. The value of I is __________. ( )。
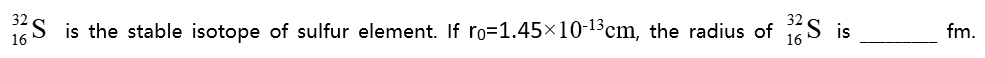 ( )。
( )。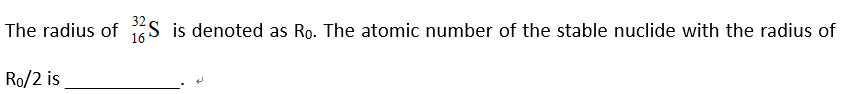 ( )
( ) ( )
( )
A:1 B:-3 C:3 D:0
答案:-3
A:
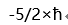 B:
B: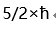 C:0
D:ћ
C:0
D:ћ答案:
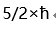
A:
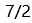 B:0
C:
B:0
C: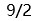 D:
D: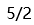
答案:
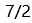
A:1.45 B:3.65 C:5.27 D:4.60
答案:4.60
A:
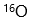 B:
B: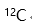 C:
C: D:
D: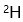
答案:

A:
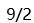 B:
B: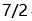 C:
C: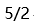 D:
D: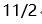
答案:
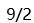
温馨提示支付 ¥3.00 元后可查看付费内容,请先翻页预览!

